Mission Statement
Discovery of HIV and increased awareness of hepatitis B and C has led OSHA to issue the Bloodborne Pathogens (BBP) Standard in 1991 to protect healthcare workers in the U.S. The BBP Standard includes the idea of universal precautions, requiring employers to provide personal protective equipments and medical safety devices such as retractable needles, and implement work practice controls, and banned recapping such as hypodermic syringes. Prior to this, physicians and dentists did not wear gloves for routine exams and treatments.
The Needlestick Safety and Prevention Act of 2000 has urged healthcare workers and the industry to seek out these medical safety devices. Hypodermic syringes, angiocath needles, and other needles for blood sampling were disposable, yet problems arose with the disposal process, and needle stick injuries were still occurring. Medical safety devices were mostly hypodermic syringes, angiocath and others, in which needles could be either retracted or re-sheathed for safe disposal.
The current dental aspirating syringe and the disposable needle have been in use for more than fifty years and have served the profession well up to now. The method of detaching needles from the syringe, i.e., recapping and unscrewing the needles, has been a problem for dental professionals for some time. Because there has been a lack of better alternatives, OSHA and CDC still recommend recapping by one handed scoop technique or using a recapping device in dentistry despite its own danger of recapping.
In a research article, it has been shown that dental assistants (75%) have sustained needle stick injuries more than dentists (7%) and hygienists (18%), and 86% of the injuries among assistants were due to the syringe needles. Dental assistants have sustained most of the injuries while cleaning instruments and trays (24%), followed by changing a local anesthetic cartridge (19%) and recapping a needle (18%). (Shah, SM. et al.; Percutaneous injuries among dental professionals in Washington State, in BMC Public Health 2006, 6:269)
Physicians and nurses routinely dispose of the medical sharps by themselves. Why do dentists delegate the disposal of the needles to assistants? Presumably, because disposing the used needles is cumbersome, non-essential, and time consuming. This is the problem associated with the current dental syringe and needle system. Recapping is not the only issue, but the whole disposal process has to be safe, simple and easy for everyone to reduce needle stick injuries.
What is the worth of your safety? When you buy the LeEject 2 syringes and needles, you are not just buying needles; you are buying safety and convenience for you and your staff.
There are dental professionals who have contracted serious diseases by needle stick injuries. I intend to help the victims with the profits from the LeEject 2 system sales since I have there myself.
Let's keep our workplace safe. Let us be more aware of potential dangers.
We are committed to reducing needle stick injuries
by designing safer dental devices, as well as through
education and prevention.
The LeEject Cartridge Syringe and Needle System
Our innovative idea of loading the needle from the side port was developed quite some time ago and initial samples were made. We first pursued attempts to license the system, but a lack of responses from manufacturers led to abandoning the idea of licensing.
After a long pause, contract manufacturing was decided upon. The search for a manufacturer who would commit to a novel and unproven product was a long process. Then, contract negotiations and building mutual trust took some time. There were numerous revisions, refinements and discussions about quality control for the syringe and needle models.
The U. S. Food and Drug Administration (FDA) application process is a long process and saw many adjustments. The basic design of the syringe was changed by adding the plastic sheath to secure the needle, the agency used for filing with the FDA was changed, and the leading examiner of this product at the FDA was replaced during the process. The claim of self aspiration was dropped during the FDA application and self-aspirating syringes are no longer available in the US market.
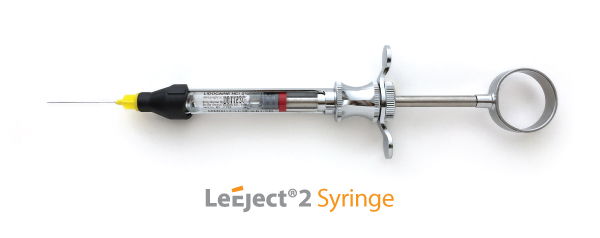
Here is the original LeEject syringe
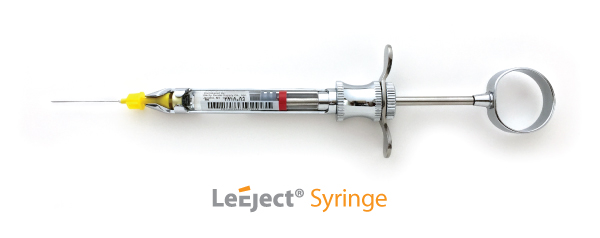
Here is the LeEject 2 syringe with the plastic sheath: